
Identified critical functionalities used by the users for OQ testing.
 Authored OQ and PQ test scripts to challenge all regulatory and security requirements. Identified key work streams for testing based on risk analysis. Liaised with lead business users (PK Analyst, QC Coordinator/QC Reviewer) and developed system process flows and User Requirements Specifications.
Authored OQ and PQ test scripts to challenge all regulatory and security requirements. Identified key work streams for testing based on risk analysis. Liaised with lead business users (PK Analyst, QC Coordinator/QC Reviewer) and developed system process flows and User Requirements Specifications.  Ensured the execution of UAT test cases and documentation of the test results. Created and tracked deviations occurred during testing process. Worked closely with Thermo team in producing and executing all required documents. Authored IQ’s for installation Watson LIMS. Developed, analyzed and reviewed test scripts to check the functionalities of the application for 21CFR part 11 compliance. Organized and maintained documents like Requirements Traceability Matrix (RTM) and Test Strategy. Designed and authored validation protocols for Watson LIMS application. Worked with Thermo team in developing Design Specification document. Authored Amgen Specific End-To-End test scenarios to test complete business process for ‘Small Molecules’ and ‘Large Molecules teams’ which involves different instruments are interfacing with Watson LIMS. Gathered business requirements and participated in the designing of the Validation Master Plan (VMP) and Testing Criteria. Translated Business requirements into user Documentations. Actively involved in discussion with ‘Small Molecules’ and ‘Large Molecules teams’ in analyzing the business requirements and convert them into functional requirements. Worked as Watson LIMS Business Analyst which is used for Pharmaco Kinetics Drug Metabolism (PKDM) studies. Project: Watson Laboratory Information Management System Validation. Microsoft Office, Documentum, Watson LIMS, Lab Vantage LIMS, Labware LIMS, SAP R/3,ELAN Excellent communication and documentation skills in the field of technical writing. Good understanding of statistical design of experiments, process capability analysis, FMEA, FTA hazard analysis, failure analysis and six sigma tools. Strong knowledge and understanding of GAMP5 and regulatory compliance issues along with current pharmaceutical industry standards. Implementation of 21 CFR Part 11 regulations. Participation in 21 CFR Part 11 compliance assessments. Expert in Requirement Analysis / System Specification Analysis. Quality and detail oriented, ability to interact with all levels of the organization and influence decision making. Knowledge of industry practices and regulatory expectations as they relate to commissioning, qualification, and validation programs. Proficient in dealing with Standard Operating Procedures (SOPs), Test plan, Laboratory information management system (LIMS). Extensive experience in preparing and executing qualification protocols (IQ, OQ, PQ). Participation in 21 CFR Part 11 and GxP compliance assessments. Knowledge and working experience in GLP, GMP and GCP suites. Creating user requirements document by interacting with the end users and developers.
Ensured the execution of UAT test cases and documentation of the test results. Created and tracked deviations occurred during testing process. Worked closely with Thermo team in producing and executing all required documents. Authored IQ’s for installation Watson LIMS. Developed, analyzed and reviewed test scripts to check the functionalities of the application for 21CFR part 11 compliance. Organized and maintained documents like Requirements Traceability Matrix (RTM) and Test Strategy. Designed and authored validation protocols for Watson LIMS application. Worked with Thermo team in developing Design Specification document. Authored Amgen Specific End-To-End test scenarios to test complete business process for ‘Small Molecules’ and ‘Large Molecules teams’ which involves different instruments are interfacing with Watson LIMS. Gathered business requirements and participated in the designing of the Validation Master Plan (VMP) and Testing Criteria. Translated Business requirements into user Documentations. Actively involved in discussion with ‘Small Molecules’ and ‘Large Molecules teams’ in analyzing the business requirements and convert them into functional requirements. Worked as Watson LIMS Business Analyst which is used for Pharmaco Kinetics Drug Metabolism (PKDM) studies. Project: Watson Laboratory Information Management System Validation. Microsoft Office, Documentum, Watson LIMS, Lab Vantage LIMS, Labware LIMS, SAP R/3,ELAN Excellent communication and documentation skills in the field of technical writing. Good understanding of statistical design of experiments, process capability analysis, FMEA, FTA hazard analysis, failure analysis and six sigma tools. Strong knowledge and understanding of GAMP5 and regulatory compliance issues along with current pharmaceutical industry standards. Implementation of 21 CFR Part 11 regulations. Participation in 21 CFR Part 11 compliance assessments. Expert in Requirement Analysis / System Specification Analysis. Quality and detail oriented, ability to interact with all levels of the organization and influence decision making. Knowledge of industry practices and regulatory expectations as they relate to commissioning, qualification, and validation programs. Proficient in dealing with Standard Operating Procedures (SOPs), Test plan, Laboratory information management system (LIMS). Extensive experience in preparing and executing qualification protocols (IQ, OQ, PQ). Participation in 21 CFR Part 11 and GxP compliance assessments. Knowledge and working experience in GLP, GMP and GCP suites. Creating user requirements document by interacting with the end users and developers. 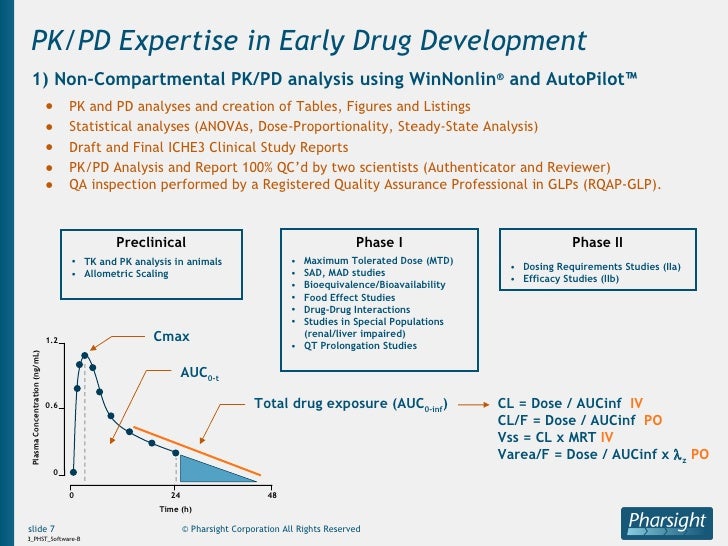
#Winnonlin user guide manual#
Thorough understanding of the Software Development Lifecycle and Validation Lifecycle with the emphasis on the manual and automated testing.Over 8 years of diversified experience as a Business Analyst in the pharmaceutical industry, known for the ability to develop and implement processes that positively impact the compliance results.







 0 kommentar(er)
0 kommentar(er)
